9.8 Chemical Cell
Quiz Summary
0 of 8 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 8 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 8
1. Question
1 point(s)Diagram 2 shows a voltaic cell.
Rajah 2 menunjukkan suatu sel volta.
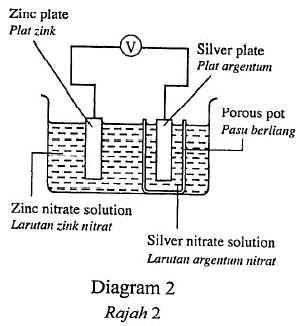
The function of the porous pot is to allow the flow ofFungsi pasu berliang adalah untuk membenarkan pengaliran
CorrectIncorrect -
Question 2 of 8
2. Question
1 point(s)Diagram 8 shows a simple chemical cell built using a lime. Two different metals are used as electrodes.
Rajah 8 menunjukkan sel kimia ringkas yang dibina menggunakan buah limau. Dua logam berlainan digunakan sebagai elektrod.
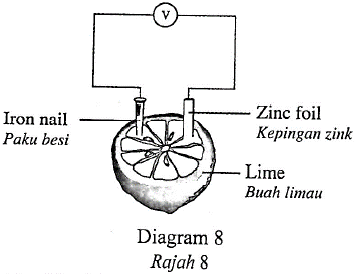
Which of the following metal can be used to replace the iron nail to obtain the highest voltage reading?
Antara logam berikut, yang manakah boleh menggantikan paku besi itu untuk mendapat bacaan voltan yang paling tinggi?
CorrectIncorrect -
Question 3 of 8
3. Question
1 point(s)Table 3 shows information about three simple voltaic cells.
Jadual 3 menunjukkan maklumat tentang tiga sel volta ringkas.
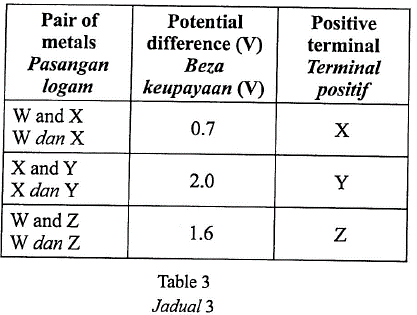
What is the potential difference of a voltaic cell which uses \(\mathrm{Y}\) and \(\mathrm{Z}\) as electrodes?
Berapakah beza keupayaan sel volta yang menggunakan \(\mathrm{Y}\) dan \(\mathrm{Z}\) sebagai elektrod?
CorrectIncorrect -
Question 4 of 8
4. Question
1 point(s)Diagram 5 shows a simple voltaic cell.
Rajah 5 menunjukkan suatu sel volta ringkas.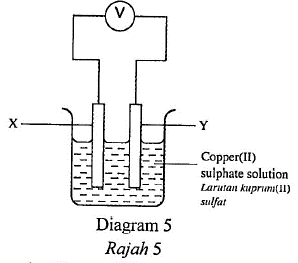
Which pair of materials are suitable to be used as electrodes \(\mathrm{X}\) and \(\mathrm{Y}\) ?
Pasangan bahan manakah yang sesuai digunakan sebagai elektrod \(\mathrm{X}\) dan \(\mathrm{Y}\) ?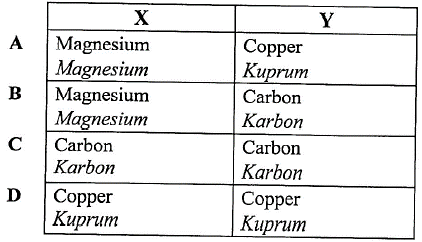 CorrectIncorrect
CorrectIncorrect -
Question 5 of 8
5. Question
1 point(s)Diagram 4 shows a simple voltaic cell.
Rajah 4 menunjukkan satu sel voltan ringkas.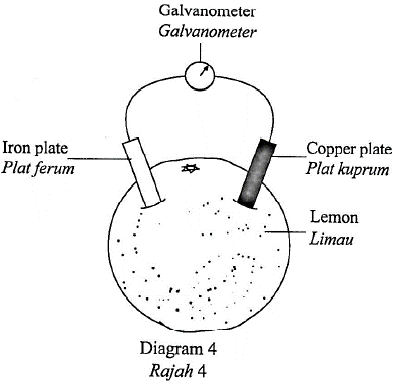
What does the needle of the galvanometer deflect?
Mengapakah jarum galvanometer terpesong?
CorrectIncorrect -
Question 6 of 8
6. Question
1 point(s)Diagram 10 shows a simple voltaic cell.
Rajah 10 menunjukkan satu sel volta ringkas.
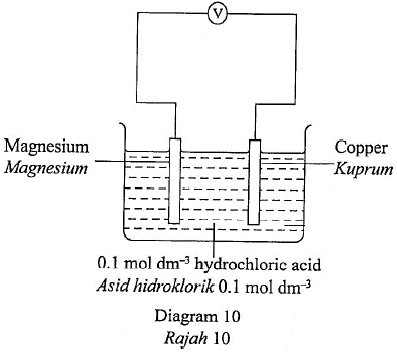
Which half equation represents the reaction at the positive terminal of the voltaic cell?
Setengah persamaan manakah mewakili tindak balas di terminal positif sel voltan?CorrectIncorrect -
Question 7 of 8
7. Question
1 point(s)Which chemical cell is not rechargeable?
Sel kimia manakah yang tidak boleh dicas semula?CorrectIncorrect -
Question 8 of 8
8. Question
1 point(s)Which pair correctly shows the differences between an electrolytic cell and a voltaic cell?
Pasangan manakah yang betul menunjukkan perbezaaan antara sel elektrolisis dengan sel voltan?
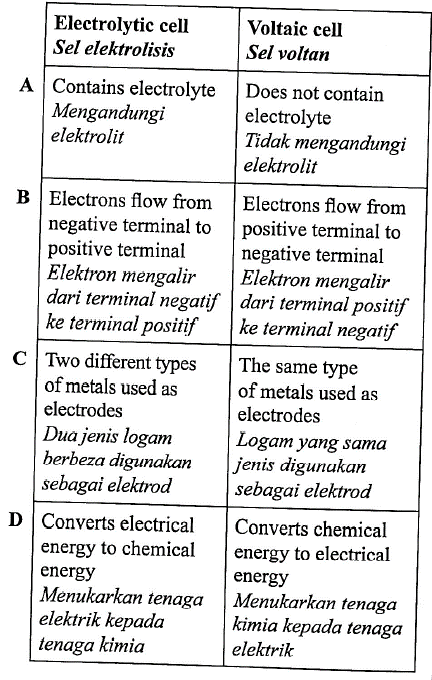 CorrectIncorrect
CorrectIncorrect