1.2.1.1 The Electrochemical Series MCQ
Quiz Summary
0 of 2 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 2 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 2
1. Question
1 point(s)Diagram 3 shows four chemical cells.
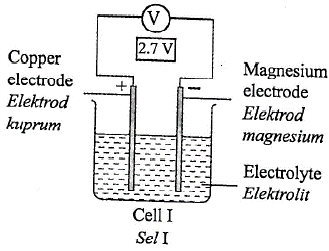
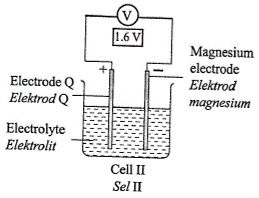
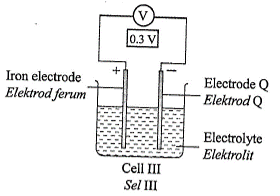
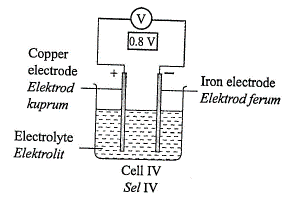 What is the arrangement of metal \(\mathrm{Q}\), copper, iron and magnesium in descending order of the electrochemical series?CorrectIncorrect
What is the arrangement of metal \(\mathrm{Q}\), copper, iron and magnesium in descending order of the electrochemical series?CorrectIncorrect -
Question 2 of 2
2. Question
1 point(s)Table below shows the observation for the reactions between metal \(\mathrm{X}\) with two different salt solutions.
Salt solution
Observation
Copper(II) sulphate
Brown deposit is formed
Zinc sulphate
No change
Which of the following is the correct descending order of metal \(\mathrm{X}\), copper and zinc in the electrochemical series?
CorrectIncorrect