6.3.1 Strength of Acids and Alkalis
Quiz Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
1 point(s)CHEM0705010
Which of the following are weak acid?CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)CHEM0705020
Which of the following statements are true for all strong acids in aqueous solutions?CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)CHEM0705030
0.5 mol of each of the following compounds was dissolved completely in 1dm³ of water. Which of the solutions would have the lowest pH value?CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)CHEM0705040
The pH value of 1mol dm⁻³ ethanoic acid, CH₃COOH is higher than the pH value of 1 mol dm⁻³ sulphuric acid, H₂SO₄. This is because the ethanoic acidCorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)CHEM0705050
Which of the following facts is correct about the acids?CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)CHEM0705060
What is the colour of methyl orange/red in acid?CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)CHEM0705070
What is the colour of phenolphthalein in alkali?CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)CHEM0705080
What is the colour of bromothymol blue in acid?CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)CHEM0705090
Why is ammonia solution a weak alkali?CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)CHEM0705100
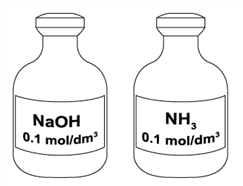
The figure above shows two aqueous solutions. Which of the following statements is true about the solutions?
CorrectIncorrect