6.4.2 Understanding Acids 2
Quiz Summary
0 of 12 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 12 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 12
1. Question
1 point(s)CHEM0702010
Which of the following substance reacts with dilute nitric acid to produce hydrogen gas?CorrectIncorrect -
Question 2 of 12
2. Question
1 point(s)CHEM0702020
Which of the following substance reacts with dilute nitric acid to produce carbon dioxide gas?CorrectIncorrect -
Question 3 of 12
3. Question
1 point(s)CHEM0702030
The figure shows the apparatus set up for an electrolysis experiment. The ammeter will show a reading when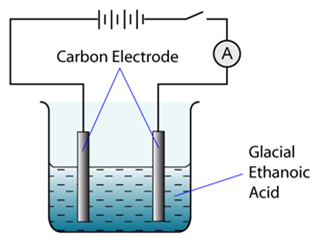 CorrectIncorrect
CorrectIncorrect -
Question 4 of 12
4. Question
1 point(s)CHEM0702040
Which of the followings are NOT true about hydrochloric acid?CorrectIncorrect -
Question 5 of 12
5. Question
1 point(s)CHEM0702050
Which of the following statements are true about the reactions of dilute hydrochloric acid, sulphuric acid and nitric acid?CorrectIncorrect -
Question 6 of 12
6. Question
1 point(s)CHEM0702060
Which of the following statements are true for all acids?CorrectIncorrect -
Question 7 of 12
7. Question
1 point(s)CHEM0702070
The acidity of hydrogen chloride gas will not shown if it dissolves in ___________.CorrectIncorrect -
Question 8 of 12
8. Question
1 point(s)CHEM0702080
Reaction Observation zinc powder + glacial ethanoic acid No changes zinc powder + glacial ethanoic acid + water Effervescence The table above shows the observation for the reaction between ethanoic acid and zinc. Which of the following explains the observation?
CorrectIncorrect -
Question 9 of 12
9. Question
1 point(s)CHEM0702090
Acids Sulphuric acid Nitric acid Concentration/mol dm⁻³ 0.01 0.01 pH 1.7 2 The table shows the concentration and pH value of two acids. Which of the following statement is true about the acids?
CorrectIncorrect -
Question 10 of 12
10. Question
1 point(s)CHEM0702100
Solution Observation P Effervescence Q No changes Calcium carbonate is added to solutions P and Q in two different test tubes. The table above shows the experimental result for solutions P and Q. Possibly, solutions P and Q are
CorrectIncorrect -
Question 11 of 12
11. Question
1 point(s)CHEM0702110
Farmers neutralize the acidity in the soil by addingCorrectIncorrect -
Question 12 of 12
12. Question
1 point(s)CHEM0702120
Baking powder contains sodium hydrogen carbonate. It reacts with acidic substances to releaseCorrectIncorrect