6.5.2 Dilution and Stoichiometry Calculation
Quiz Summary
0 of 10 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 10 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 10
1. Question
1 point(s)CHEM0708010
You are asked to prepare 250 cm³ of the aqueous sodium hydroxide solution of concentration 0.5 moldm⁻³. Find the mass of sodium hydroxide that you need to dissolve into the solution.
[ Relative atomic mass: Na=23, O = 16; H=1]CorrectIncorrect -
Question 2 of 10
2. Question
1 point(s)CHEM0708020
Some distilled water is added into 50cm³ of hydrochloric acid 2M to dilute it to 0.2M. Find the volume of the dilute hydrochloric acid obtained.CorrectIncorrect -
Question 3 of 10
3. Question
1 point(s)CHEM0708030
25 cm³ of 0.5 mol sodium hydroxide solution is diluted with water until its volume becomes 100cm³. What is the concentration of the solution produced?CorrectIncorrect -
Question 4 of 10
4. Question
1 point(s)CHEM0708040
2K + CuSO₄ → K₂SO₄ + Cu
The equation above represents the reaction between potassium and copper(II) sulphate solution. Find the volume of 0.5 mol/dm³ copper(II) sulphate solution needed to completely react with 3.51g potassium. [Relative Atomic Mass of Potassium = 39]
CorrectIncorrect -
Question 5 of 10
5. Question
1 point(s)CHEM0708050
The following chemical equation represents the neutralization reaction between sulphuric acid and sodium hydroxide solution.H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
20.00 cm³ of 0.1 mol/dm³ sodium hydroxide solution was titrated with 0.1 mol dm-3 sulphuric acid. What is the final reading of the burette if the initial reading is 5.00 cm³?
CorrectIncorrect -
Question 6 of 10
6. Question
1 point(s)CHEM0708060
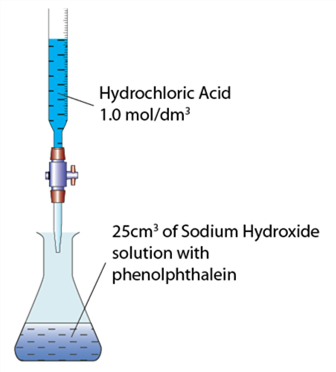
The figure shows the set-up of the apparatus for the titration of sodium hydroxide solution 0.5 mol/dm3 with hydrochloric acid. What is the total volume of the mixture in the conical flask at the endpoint?
CorrectIncorrect -
Question 7 of 10
7. Question
1 point(s)CHEM0708070
The equation below represents the neutralization reaction between nitric acid and an alkali X(OH)₂.2HNO₃ + X(OH)₂ → X(NO₃)₂ + 2H₂O
Given that 25 cm3 of the alkali 0.5 mol dm-3 fully neutralises 25 cm3 of nitric acid. What is the concentration of the nitric acid?
CorrectIncorrect -
Question 8 of 10
8. Question
1 point(s)CHEM0708080
The following equation represents the reaction between nitric acid with excess magnesium.Mg + 2HNO₃ → Zn(NO₃)₂ + H₂
If 20.0 g of magnesium reacts with 100 cm3 of 2.0 mol/dm³ nitric acid, find the mass of the unreacted magnesium? (Relative atomic mass of Mg = 24).
CorrectIncorrect -
Question 9 of 10
9. Question
1 point(s)CHEM0708090
11.50 g of sodium burnt in excess oxygen gas to produce sodium oxide. The product is then dissolved in 250.0 cm³ water to produce sodium hydroxide solution. What is the molarity of the sodium hydroxide solution? [Relative atomic mass: Na = 23]CorrectIncorrect -
Question 10 of 10
10. Question
1 point(s)CHEM0708100
The following chemical equation represents a reaction between zinc oxide and hydrochloric acid.ZnO + 2HCl → ZnCl₂ + H₂O
What is the mass of zinc chloride formed when 24.3g of zinc oxide powder is reacted with excess hydrochloric acid? (Molar mass : ZnO = 81g/mol; ZnCl₂ = 136g/mol)
CorrectIncorrect