7.2.1 Factors Affecting the Rate of Reaction
Quiz Summary
0 of 11 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 11 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 11
1. Question
1 point(s)CHEM1004010
Which of the following properties of a reactant will give effect to the rate of a reaction?CorrectIncorrect -
Question 2 of 11
2. Question
1 point(s)Which of the following factors will affect the rate of reaction that involve gases as a reactant?
CorrectIncorrect -
Question 3 of 11
3. Question
1 point(s)In a chemical reaction, some granules of zinc react with dilute hydrochloric acid to produce salt and hydrogen gas. The release of hydrogen gas can be faster if
CorrectIncorrect -
Question 4 of 11
4. Question
1 point(s)Hydrogen peroxide solution is decomposed by using manganese (IV) oxide as catalyst. Three experiments were carried out using a fixed amount of manganese (IV) oxide. The results are shown in the graph. The solutions used were :
(a) 25 cm³ of 2.0 mol/dm³ hydrogen peroxide;
(b) 50 cm³ of 1.0 mol/dm³ hydrogen peroxide;
(c) 50 cm³ of 2.0 mol/dm³ hydrogen peroxide;Which curve did each solution pro-duce?
CorrectIncorrect -
Question 5 of 11
5. Question
1 point(s)The equation below shows a reaction to produce hydrogen gas.
3H2SO4 + 2Al → Al2(SO4)3 + 3H2
Which of the following will increase the rate of the reaction?
CorrectIncorrect -
Question 6 of 11
6. Question
1 point(s)In a reaction between iron chips and nitric acid, the rate of reaction decreases with time. This is because
CorrectIncorrect -
Question 7 of 11
7. Question
1 point(s)2H2O2 → 2H2O + O2
The equation above show the decomposition of hydrogen peroxide. The rate of this reaction decreases over time because
CorrectIncorrect -
Question 8 of 11
8. Question
1 point(s)2 experiments are carried out to study the rate of reaction between calcium carbonate and nitric acid to produce carbon dioxide gas. The quantity of reactant used are as follow:
Experiment 1: Excess calcium carbonate and 25 cm³ of 1 mol/dm³ nitric acid
Experiment 2: Excess calcium carbonate and 50 cm³ of 0.5 mol/dm³ nitric acidWhich of the following graphs represents the 2 experiments
CorrectIncorrect -
Question 9 of 11
9. Question
1 point(s)Which of the following are the characteristics of a catalyst?
CorrectIncorrect -
Question 10 of 11
10. Question
1 point(s)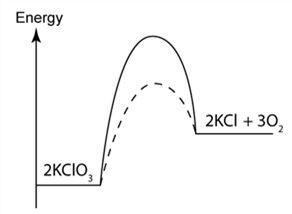
The figure shows the energy profile diagram for the decomposition reaction of Potassium chlorate(V). Which of the following is true about curve P and Q?
CorrectIncorrect -
Question 11 of 11
11. Question
1 point(s)Which of the following acid produces the highest rate of reaction when reacts with some limestone?
CorrectIncorrect