6.3 Strength of Acids and Alkali
2 Topics | 1 Quiz
6.4 Chemical Properties of Acids and Alkalis
5 Topics | 4 Quizzes
6.5 Concentration of Aqueous Solution
6 Topics | 2 Quizzes
6.7 Neutralisation
1 Topic
6.8 Salts, Crystals and Their Uses in Daily Life
3 Topics | 1 Quiz
6.9 Preparation of Salts
5 Topics | 2 Quizzes
6.10 Effect sof Heat on Salts
3 Topics
6.11 Qualitative Analysis
6 Topics | 2 Quizzes
07 Rate of Reaction
7.1 Determining Rate of Reaction
5 Topics | 3 Quizzes
7.2 Factors Affecting Rate of Reactions
7 Topics | 1 Quiz
7.4 Collision Theory
2 Topics | 1 Quiz
08 Manufacture Substances in Industries
8.1 Alloy and Its Importance
6 Topics | 1 Quiz
8.2 Composition of Glass and Its Uses
2 Topics | 1 Quiz
8.4 Composite Materials and Its Importance
2 Topics | 1 Quiz
7.2.5 Presence of Catalyst
Presence of Catalyst
- A catalyst is a substance which can change the rate of reaction.
- There are 2 types of catalyst:
- Positive catalyst – Increase the rate of reaction.
- Negative catalyst – Reduce the rate of reaction.
Experiment 1
Set 1: Zinc + Hydrochloric Acid
Set 2: Zinc + Hydrochloric Acid + Copper Sulphate (Catalyst)
Chemical Reaction:
Zn + 2HCl → ZnCl2 + H2
Result:
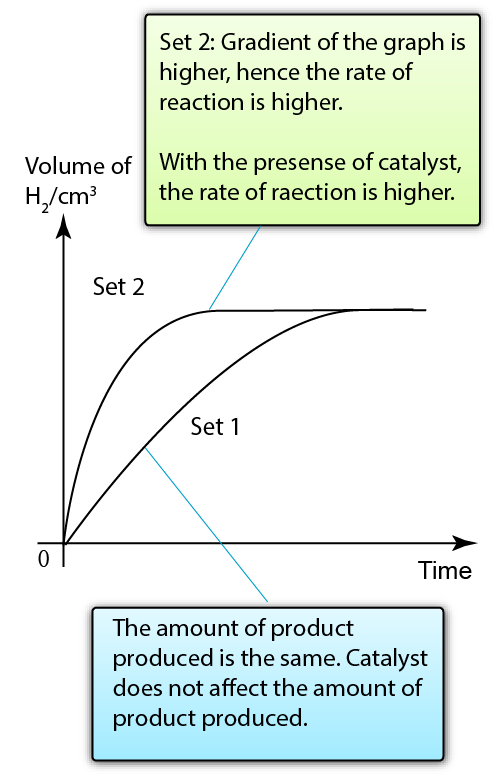
Copper(II) sulphate acts as a catalyst to increase the rate of reaction between zinc and hydrochloric acid
Conclusion
The presence of catalyst increases the rate of reaction.
Experiment 2
Set 1: Decomposition of Hydrogen Peroxide
Set 2: Decomposition of Hydrogen Peroxide + Manganese(IV) Oxide (Catalyst)
Chemical Reaction:
2H2O2 → 2H2O + O2
Result:
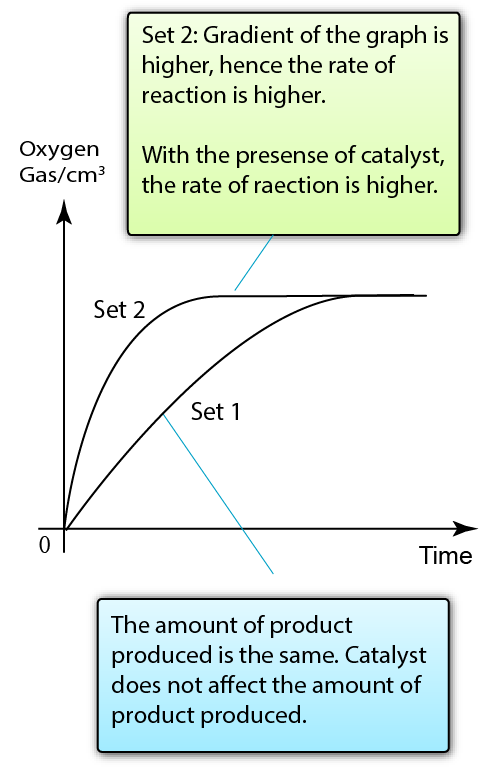
Manganese(IV) oxide acts as a catalyst to increase the rate of reaction between zinc and hydrochloric acid
Conclusion:
The presence of catalyst increases the rate of reaction
Note:
In SPM, you need to remember the catalyst used in both the chemical reaction above.
Characteristic of Catalyst
- A catalyst is a substance which can change the rate of reaction.
- There are a few things you need to know about catalyst:
- Chemically, the catalyst remains unchanged during a reaction.
- Catalyst does not change the quantity of the product.
- Catalyst is specific, which means different chemical reaction may have different catalyst.
- Just a small amount needed to achieve a big increase in the rate of reaction.
- More amount of catalyst used can further increase the rate of reaction.
- Catalyst in powder form can further increase the rate of reaction.
- Catalyst may undergo physical change in a reaction.
List of Reactions and the Catalyst
| Chemical Reaction | Catalyst |
|---|---|
| Decomposition of hydrogen peroxide: 2H2O2 → 2H2O + O2 | Manganese(IV) oxide, MnO2 Lead(II) oxide, PbO Lead(IV) oxide, PbO2 |
| Reaction between Zinc and Hydrochloric Acid: Zn + 2HCl → ZnCl2 + H2 | Manganese (IV) oxide, MnO2 Copper (II) oxide, CuO Zinc Oxide, ZnO Silicon (IV) oxide, SiO2 |
| Decomposition of Potassium Chlorate (V): 2KClO3 + 2KCl → 3O2 | Copper (II) sulphate, CuSO4 Copper (II) chloride, CuCl2 Copper (II) nitrate, Cu(NO3)2 |
| Haber Process N2 + 3H2 → 2NH3 | Iron |
| Contact Process 2SO2 + O2 → 2SO3 | Vanadium(V) oxide, V2O5 |
| Ostwald Process 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(1) | Platinum |