9.2 Rusting as Redox Reaction
Quiz Summary
0 of 6 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 6 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 6
1. Question
1 point(s)Iron rusts in the presence of oxygen and water.
Which method causes iron to rust faster?
Besi berkarat dengan kehadiran oksigen dan air.
Kaedah manakah menyebabkan besi berkarat lebih cepat?
CorrectIncorrect -
Question 2 of 6
2. Question
1 point(s)Diagram 14 shows a water droplet on a piece of iron.
Rajah 14 menunjukkan setitis air di atas sebatang besi.
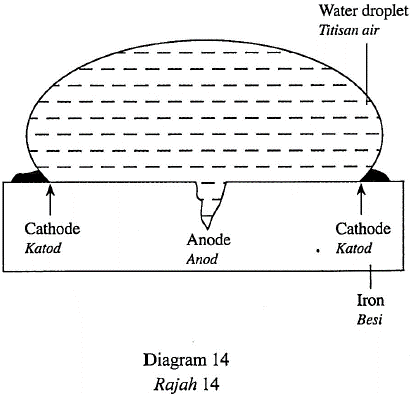
Which equation occurs at the cathode?
Persamaan manakah yang berlaku di katod?CorrectIncorrect -
Question 3 of 6
3. Question
1 point(s)A student found that an iron spoon rusts easily. What is the suitable method to solve the problem?
Seorang pelajar mendapati sudu besi mudah berkarat. Apakah kaedah yang sesuai untuk menyelesaikan masalah itu?
CorrectIncorrect -
Question 4 of 6
4. Question
1 point(s)A method to control the rusting of underground iron pipelines is through sacrificial protection. Which of the following is the sacrificial metal?
Cara mengawal pengaratan saluran paip besi bawah tanah adalah melalui perlindungan korban. Antara yang berikut, yang manakah adalah logam korban?
CorrectIncorrect -
Question 5 of 6
5. Question
1 point(s)Which substance is suitable to change \(\mathrm{Fe}^{2+}\) ions to \(\mathrm{Fe}^{3+}\) ions?
Bahan manakah yang sesuai untuk menukarkan ion \(\mathrm{Fe}^{2+}\) kepada ion \(\mathrm{Fe}^{3+}\) ?
CorrectIncorrect -
Question 6 of 6
6. Question
1 point(s)Diagram 8 shows a ship in the sea that uses sacrificial anode method to prevent rusting. Metal X becomes thinner after several weeks.
Rajah 8 menunjukkan sebuah kapal di laut yang menggunakan kaedah anod korban bagi mengelakkan pengaratan.
Logam X menjadi semakin nipis selepas beberapa minggu.
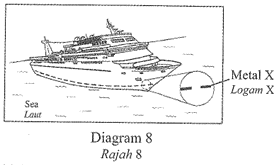
Which of the following explains the situation?
Antara yang berikut, yang manakah menerangkan situasi tersebut?
CorrectIncorrect