01 Introduction to Chemistry
02 Matter and the Atomic Structure
2.1 Basic Concepts of Matter
7 Topics | 2 Quizzes
2.3 Atomic Structure
5 Topics | 2 Quizzes
2.4 Isotopes and Its Uses
2 Topics | 1 Quiz
03 The Mole Concept, Chemical Formula and Equation
3.1 Relative Atomic Mass and Relative Molecular Mass
3 Topics | 1 Quiz
3.2 Mole Concept
6 Topics | 4 Quizzes
3.3 Chemical Formula
7 Topics | 1 Quiz
3.4 Chemical Equation
2 Topics | 1 Quiz
04 The Periodic Table of Elements
4.1 Introduction to Periodic Table
5 Topics | 1 Quiz
4.2 Group 18 Elements
2 Topics | 1 Quiz
4.3 Group 1 Elements
6 Topics | 1 Quiz
4.4 Group 17 Elements
7 Topics | 1 Quiz
4.5 Period and Transition Metal
4 Topics | 1 Quiz
05 Chemical Bond
5.1 Basics of Compound Formation
6 Topics | 2 Quizzes
5.2 Ionic Bond
3 Topics | 1 Quiz
5.3 Covalent Bond
4 Topics | 1 Quiz
06 Acids, Bases and Salts
6.2 pH Value
2 Topics
2.1.2 Three States of Matter
Matter exists in 3 states of matter, namely, solid state, liquid state and gaseous state.
Characteristics of Matter in Solid, Liquid and Gaseous State
Arrangement of Particles
| Solid |
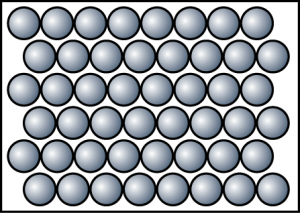 |
| Particles are arranged in an orderly manner and close to one another. |
| Liquid |
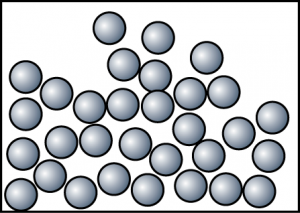 |
| Particles are not arranged in order. The space between particles is moderately large. |
| Gas |
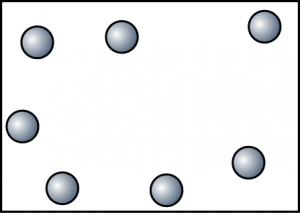 |
| The particles are very far apart and randomly arranged. |
Movement of Particles
| Solid | Particles vibrate at fixed positions. |
| Liquid | Particles move randomly and slowly and sometimes will collide against each other. |
| Gas | The particles move randomly in all directions at great speed. |
The force of Attraction Between Particles
| Solid | very strong |
| Liquid | Strong but weaker than in the solid state. |
| Gas | very weak |
Ability to be compressed
| Solid | Very difficult to be compressed because the particles are packed closely. |
| Liquid | Not easily compressed because the particles are packed quite closely. |
| Gas | Easily compressed because the particles are very far apart. |
Heat Energy content
| Solid | Lowest Energy Content |
| Liquid | Moderate energy content. |
| Gas | Highest energy content |
Volume and Shape
| Volume | Shape | |
| Solid | Fixed | Fixed |
| Liquid | Fixed | Follows the container |
| Gas | Follows the container | Fills the whole container |