01 Introduction to Chemistry
02 Matter and the Atomic Structure
2.1 Basic Concepts of Matter
7 Topics | 2 Quizzes
2.3 Atomic Structure
5 Topics | 2 Quizzes
2.4 Isotopes and Its Uses
2 Topics | 1 Quiz
03 The Mole Concept, Chemical Formula and Equation
3.1 Relative Atomic Mass and Relative Molecular Mass
3 Topics | 1 Quiz
3.2 Mole Concept
6 Topics | 4 Quizzes
3.3 Chemical Formula
7 Topics | 1 Quiz
3.4 Chemical Equation
2 Topics | 1 Quiz
04 The Periodic Table of Elements
4.1 Introduction to Periodic Table
5 Topics | 1 Quiz
4.2 Group 18 Elements
2 Topics | 1 Quiz
4.3 Group 1 Elements
6 Topics | 1 Quiz
4.4 Group 17 Elements
7 Topics | 1 Quiz
4.5 Period and Transition Metal
4 Topics | 1 Quiz
05 Chemical Bond
5.1 Basics of Compound Formation
6 Topics | 2 Quizzes
5.2 Ionic Bond
3 Topics | 1 Quiz
5.3 Covalent Bond
4 Topics | 1 Quiz
06 Acids, Bases and Salts
6.2 pH Value
2 Topics
2.1.1 Element and Compound
Element and Compounds
Matter can be divided into elements and compounds.
Elements
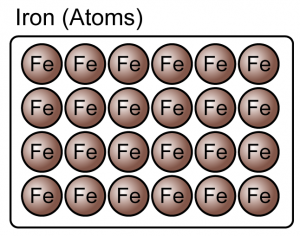
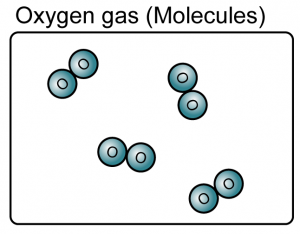
- An element is a substance that consists of only one type of atom.
- Element can be either atoms or molecules.
Example:
(Both the iron and oxygen are element because they consist of only one type of atoms)
Compounds
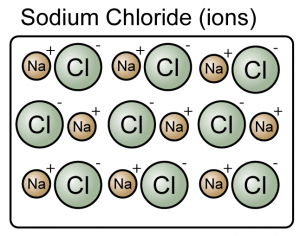
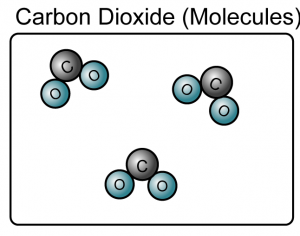
- A compound is a substance composed of molecules made up of atoms of two or more elements.
- A compound is made up of either molecules or ions.
Example:
(Both the sodium chloride and carbon dioxide are compound because they consist of more than one type of atoms)