3.2.1 Concept of Mole
Quiz Summary
0 of 7 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 7 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 7
1. Question
1 point(s)CHEM0302010
One mole of a substance is defined as the quantity of a substance that contains the same number of particles in 12.000g of _____________.
CorrectIncorrect -
Question 2 of 7
2. Question
1 point(s)CHEM0302020
Which contains more atoms, 1 mol of helium or 1 mol of uranium?
[ RAM: He=4; U=238 ]CorrectIncorrect -
Question 3 of 7
3. Question
1 point(s)CHEM0302030
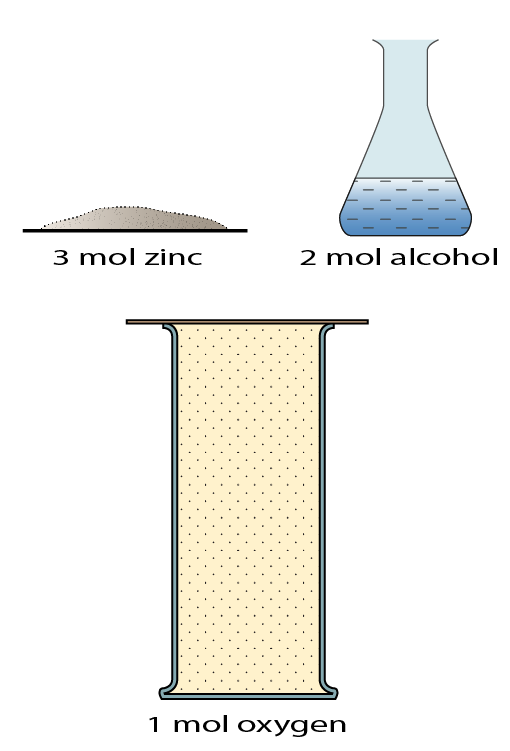
Which of the followings are true about the substance in the diagram above?
CorrectIncorrect -
Question 4 of 7
4. Question
1 point(s)CHEM0302040
Find the number of atoms in 0.3 mol of copper metal.
[Avogadro constant = 6.02 x 10²³]CorrectIncorrect -
Question 5 of 7
5. Question
1 point(s)CHEM0302050
How many moles of magnesium that contain 2.408 x 10²³ of magnesium atom? [Avogadro number = 6.02 x 10²³]
CorrectIncorrect -
Question 6 of 7
6. Question
1 point(s)CHEM0302060
Calculate the number of atoms in 0.5 mol of ammonia molecules. [Avogadro number = 6.02 x 10²³]
CorrectIncorrect -
Question 7 of 7
7. Question
1 point(s)CHEM0302070
Which of the following is correct about the Avogadro constant?
CorrectIncorrect