01 Introduction to Chemistry
02 Matter and the Atomic Structure
2.1 Basic Concepts of Matter
7 Topics | 2 Quizzes
2.3 Atomic Structure
5 Topics | 2 Quizzes
2.4 Isotopes and Its Uses
2 Topics | 1 Quiz
03 The Mole Concept, Chemical Formula and Equation
3.1 Relative Atomic Mass and Relative Molecular Mass
3 Topics | 1 Quiz
3.2 Mole Concept
6 Topics | 4 Quizzes
3.3 Chemical Formula
7 Topics | 1 Quiz
3.4 Chemical Equation
2 Topics | 1 Quiz
04 The Periodic Table of Elements
4.1 Introduction to Periodic Table
5 Topics | 1 Quiz
4.2 Group 18 Elements
2 Topics | 1 Quiz
4.3 Group 1 Elements
6 Topics | 1 Quiz
4.4 Group 17 Elements
7 Topics | 1 Quiz
4.5 Period and Transition Metal
4 Topics | 1 Quiz
05 Chemical Bond
5.1 Basics of Compound Formation
6 Topics | 2 Quizzes
5.2 Ionic Bond
3 Topics | 1 Quiz
5.3 Covalent Bond
4 Topics | 1 Quiz
06 Acids, Bases and Salts
6.2 pH Value
2 Topics
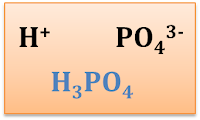
3.3.5 Formula of Ionic Compounds
Chemical Formlula of Ionic Compounds
2 Requirements to form the formula of an ionic compound
- Have at least 2 types of ions that contain opposite charge.
- The amount of positive charge/charges must be equal to the amount of negative charge/charges in the compound.
Example 1 – If the Charge of the Positive Ions = Charge of Negative Ions
Write the formula of each of the following compound
- Potassium bromide
- Sodium chloride
Example 2 – If the Charge of the Positive Ions ≠ Charge of Negative Ions
Write the formula of each of the following compound
- Calcium iodide
- Sodium oxide
Example 3 – If there is more than 1 element in the ion
Write the formula of each of the compound
- Ammonium sulphate
- Zinc nitrate