01 Introduction to Chemistry
02 Matter and the Atomic Structure
2.1 Basic Concepts of Matter
7 Topics | 2 Quizzes
2.3 Atomic Structure
5 Topics | 2 Quizzes
2.4 Isotopes and Its Uses
2 Topics | 1 Quiz
03 The Mole Concept, Chemical Formula and Equation
3.1 Relative Atomic Mass and Relative Molecular Mass
3 Topics | 1 Quiz
3.2 Mole Concept
6 Topics | 4 Quizzes
3.3 Chemical Formula
7 Topics | 1 Quiz
3.4 Chemical Equation
2 Topics | 1 Quiz
04 The Periodic Table of Elements
4.1 Introduction to Periodic Table
5 Topics | 1 Quiz
4.2 Group 18 Elements
2 Topics | 1 Quiz
4.3 Group 1 Elements
6 Topics | 1 Quiz
4.4 Group 17 Elements
7 Topics | 1 Quiz
4.5 Period and Transition Metal
4 Topics | 1 Quiz
05 Chemical Bond
5.1 Basics of Compound Formation
6 Topics | 2 Quizzes
5.2 Ionic Bond
3 Topics | 1 Quiz
5.3 Covalent Bond
4 Topics | 1 Quiz
06 Acids, Bases and Salts
6.2 pH Value
2 Topics
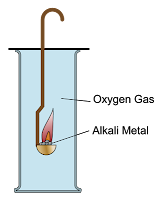
4.3.4 Reaction of Alkali Metals with Oxygen
Alkali Metals React with Oxygen
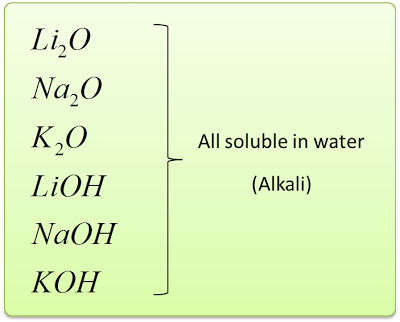
- Group 1 metals react with oxygen gas produces metal oxides. These metal oxides dissolve in water produces alkalis.
Group 1 Metals + Oxygen Gas → Metal Oxide
- Lithium, sodium and potassium form white oxide powders after reacting with oxygen.
- The white powder is the oxide of lithium, sodium and potassium.
- When the white powder is dissolved in water, it produces a solution which turned red litmus paper blue. Which means, these oxides dissolve in water to form strong alkali.
- The reactivity increases down the group from lithium, sodium to potassium.
Example
Lithium + Oxygen
4Li + O2→ 2Li2O
Dissolve in water
Li2O + H2O → 2LiOH
Observation
Lithium burns with red flame and produces white powder immediately after reaction.
Sodium + Oxygen
4Na + O2→ 2Na2O
Dissolve in water
Na2O + H2O → 2NaOH
Observation
Sodium burned with bright yellow flame, forming white powder immediately after reaction.
Potassium + Oxygen
4K + O2→ 2K2O
Dissolve in water
K2O + H2O → 2KOH
Observation
Potassium burned with very bright purplish flame, forming white powder immediately after reaction.