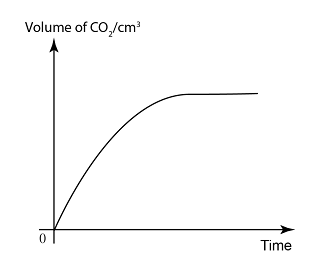
Graph of Product/Reactant Change Against Time
- In a chemical reaction,
- the reactants will decrease over time
- the product will increase over time.
- the rate of reaction will decrease over time owing to the decrease in concentration and total surface area of reactants.
- In a graph of quantity of product/reactant over time, the rate of reaction is equal to the gradient of the graph.
Example
The reaction between dilute hydrochloric acid and excess marble will produce calcium chloride and gas of carbon dioxide. Sketch the graph of
- the mass of the marble against time.
- the volume of carbon dioxide against time.
- the concentration of hydrochloric acid against time.
- the concentration of calcium chloride against time.
Answer:
a.
b.
c.
d.