!2.2.2 Using Barometer to find atmospheric pressure
Quiz Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
1 point(s)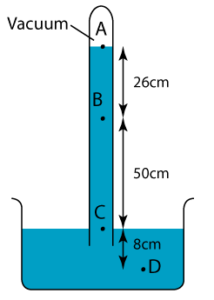
The diagram shows a simple barometer filled with mercury. What is the pressure at point A?
CorrectIncorrect -
Question 2 of 15
2. Question
1 point(s)
The diagram shows a simple barometer filled with mercury. What is the pressure at point B?
CorrectIncorrect -
Question 3 of 15
3. Question
1 point(s)
The diagram shows a simple barometer filled with mercury. What is the pressure at point C?
CorrectIncorrect -
Question 4 of 15
4. Question
1 point(s)
The diagram shows a simple barometer filled with mercury. What is the pressure at point D?
CorrectIncorrect -
Question 5 of 15
5. Question
1 point(s)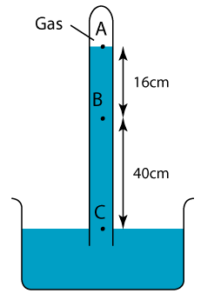
The diagram shows a simple barometer filled with mercury. Given that the pressure of the gas trapped in the mercury is equal to 20cmHg. Find the pressure at point A.
CorrectIncorrect -
Question 6 of 15
6. Question
1 point(s)
The diagram shows a simple barometer filled with mercury. Given that the pressure of the gas trapped in the mercury is equal to 20cmHg. Find the pressure at point B.
CorrectIncorrect -
Question 7 of 15
7. Question
1 point(s)
The diagram shows a simple barometer filled with mercury. Given that the pressure of the gas trapped in the mercury is equal to 20cmHg. Find the pressure at point C.
CorrectIncorrect -
Question 8 of 15
8. Question
1 point(s)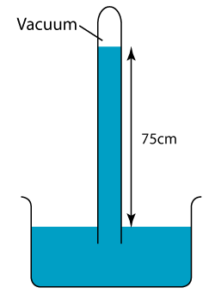
The diagram shows a simple barometer filled with mercury placed in a classroom. Find the atmospheric pressure of the classroom.
CorrectIncorrect -
Question 9 of 15
9. Question
1 point(s)
The diagram shows a simple barometer filled with mercury placed in a classroom. Find the atmospheric pressure of the classroom. (Density of mercury = 13,600 kg/m3)
CorrectIncorrect -
Question 10 of 15
10. Question
1 point(s)Given that the atmospheric pressure at Genting Highland is 72cmHg. Estimate the equivalent value of this pressure in the unit of Pascal (Pa). [density of mercury = 13,600 kgm-3]
CorrectIncorrect -
Question 11 of 15
11. Question
1 point(s)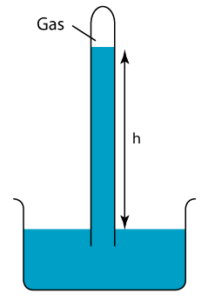
The diagram shows a simple barometer filled with mercury placed in a classroom. Given that the pressure of the gas trapped in the mercury is equal to 20cmHg and the atmospheric pressure is equal to 76 cmHg. Find the height of the mercury column, h.
CorrectIncorrect -
Question 12 of 15
12. Question
1 point(s)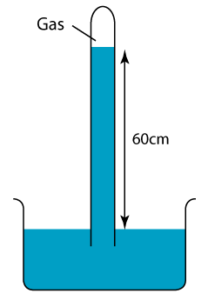
The diagram shows a simple barometer filled with mercury placed in a classroom. Find the pressure of the gas trapped in the mercury (Atmospheric pressure = 76 cmHg)
CorrectIncorrect -
Question 13 of 15
13. Question
1 point(s)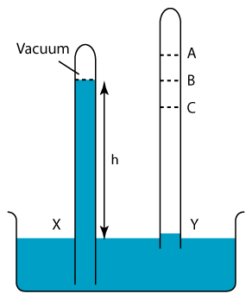
In the figure on the right, tube X shows the correct height of the mercury column. Find the level of the mercury column of tube Y.
CorrectIncorrect -
Question 14 of 15
14. Question
1 point(s)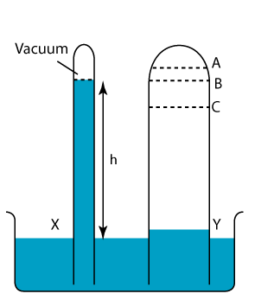
In the figure on the right, tube X shows the correct height of the mercury column. Find the level of the mercury column of tube Y
CorrectIncorrect -
Question 15 of 15
15. Question
1 point(s)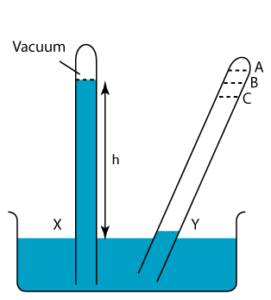
In the figure on the right, tube X shows the correct height of the mercury column. Find the level of the mercury column of tube Y.
CorrectIncorrect