!4.3.1 Specific Latent Heat
Quiz Summary
0 of 3 Questions completed
Questions:
Information
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
Results
Results
0 of 3 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
- 1
- 2
- 3
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 3
1. Question
1 point(s)A water take a shorter time to boil at a region of high altitude (Such as Genting Highland) compare with a region of low altitude although same amount of energy is used. This is because
CorrectIncorrect -
Question 2 of 3
2. Question
1 point(s)Given that the boiling point of pure water is 100°C at room temperature and pressure. What will happen to the boiling point when some salt is added to the water?
CorrectIncorrect -
Question 3 of 3
3. Question
1 point(s)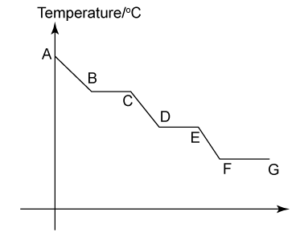
The graphs shows the cooling curve of a substance from gaseous state to solid state. At which stage the substance exist in both the liquid state and the solid state?
CorrectIncorrect