0 of 10 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 10 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) Find the oxidation number of sulphur in Na₂S₂O3. What is the oxidation number of sulphur in K₂SO₃? What is the oxidation number of vanadium in V₂O₅? What is the oxidation state of nitrogen in a nitric acid molecule? What is the oxidation state of phosphorus in a phosphoric acid molecule? What is the oxidation number of hydrogen in magnesium hydride, \(\mathrm{MgH}_2\) ? Which of the underlined element in the following compounds has the highest oxidation number? Which of the following is true about the oxidation number of the elements in NH₄ClO₃. What is the oxidation number of \(\mathrm{X}\) in \(\mathrm{XO}_4^{-}\)? What is the oxidation number of the chromium element in potassium dichromate(VI), \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) ?
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Average score
Your score
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
3. Question
1 point(s)
4. Question
1 point(s)
5. Question
1 point(s)
6. Question
1 point(s)
7. Question
1 point(s)
8. Question
1 point(s)
9. Question
1 point(s)
10. Question
1 point(s)
0 of 7 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 7 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) The following ionic equation represents the reaction between acidified potassium dichromate(VI) solution and iron(II) sulphate solution. The following equation represents the oxidation reaction between ethanol and acidified potassium dichromate(VI). What is the change in the oxidation number of the element chromium in this reaction? Diagram 6 shows the chemical formula of a compound. \begin{align} The following chemical equation represents the extraction of silicon from quartz using coke. The following ionic equation shows the reaction between zinc and acid. Which of the following IUPAC names are correct for the respective formulae of substances? The equation below shows the decomposition of potassium chlorate. 2KClO₃ → 2KCl + 3O₂ Which of the following shows the change of oxidation number of chlorine in the reaction?
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
\[
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{Fe}^{2+} \rightarrow 6 \mathrm{Fe}^{3+}+2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}
\]
What is the change of oxidation number of chromium in the reaction?
2. Question
1 point(s)
\[
\begin{aligned}
& 3 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+2 \mathrm{~K}_2 \mathrm{Cr}_2 \mathrm{O}_7+8 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \\
& \quad 3 \mathrm{CH}_3 \mathrm{COOH}+2 \mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3+2 \mathrm{~K}_2 \mathrm{SO}_4+11 \mathrm{H}_2 \mathrm{O}
\end{aligned}
\]
3. Question
1 point(s)
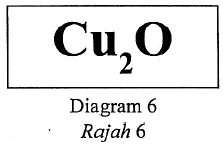
the IUPAC name and oxidation number of copper element in the compound?
\begin{array}{|c|l|c|}
\hline & \text { Name of compound } & \begin{array}{l}
\text { Oxidation number of } \\
\text { copper element }
\end{array} \\
\hline \text { A } & \text { Copper(I) oxide } & +1 \\
\hline \text { B } & \text { Copper(I) oxide } & +2 \\
\hline \text { C } & \text { Copper(II) oxide } & +1 \\
\hline \text { D } & \text { Copper(II) oxide } & +2 \\
\hline
\end{array}
\end{align}
4. Question
1 point(s)
\[
\mathrm{SiO}_2+\mathrm{C} \longrightarrow \mathrm{Si}+\mathrm{CO}_2
\]
What is the change in oxidation number of silicon?
5. Question
1 point(s)
\[
\mathrm{Zn}(\mathrm{s})+2 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})
\]
What is the change in oxidation number of hydrogen?
6. Question
1 point(s)
7. Question
1 point(s)
0 of 9 Questions completed Questions: You have already completed the quiz before. Hence you can not start it again.
Quiz is loading… You must sign in or sign up to start the quiz. You must first complete the following:
0 of 9 Questions answered correctly
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0) The equation represents the redox reaction between ammonia and copper(II) oxide. 2NH₃ + 3CuO → N₂ + 3Cu + 3H₂O Which of the following is true about the reaction? The oxidation number of calcium increases when Which of the underlined substances in the following equations undergo oxidation? Which of the following statements about a chemical reaction are true? A substance undergoes oxidation if Which of the following indicates that a substance undergoes reduction? Which of the following is NOT true about oxidation and reduction? Which of the underlined substances in the following equations undergo oxidation? The half equation represents the reduction reaction of acidified potassium dichromate(VI) solution. \begin{align}
Quiz Summary
Information
Results
Results
0 Essay(s) Pending (Possible Point(s): 0)
Average score
Your score
Categories
Pos.
Name
Entered on
Points
Result
Table is loading
No data available
1. Question
1 point(s)
2. Question
1 point(s)
3. Question
1 point(s)
4. Question
1 point(s)
5. Question
1 point(s)
6. Question
1 point(s)
7. Question
1 point(s)
8. Question
1 point(s)
9. Question
1 point(s)
\[
\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{xH}^{+}+\mathrm{ye}^{-} \longrightarrow \mathrm{zCr}^{3+}+7 \mathrm{H}_2 \mathrm{O}
\]
What are the value of \(x, y\) and \(z\) ?
\begin{array}{|c|c|c|c|}
\hline & x & y & z \\
\hline \text { A } & 14 & 6 & 2 \\
\hline \text { B } & 14 & 5 & 1 \\
\hline \text { C } & 7 & 2 & 1 \\
\hline \text { D } & 7 & 1 & 2 \\
\hline
\end{array}
\end{align}