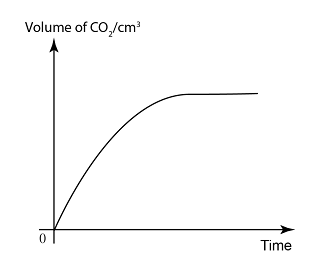
Rate of reaction is a measure of how fast a reaction occur, or how much the reactant/product change in a period of time.
Rates of reaction = Quantity change of reactants/products Total time for the reaction
Example
In a chemical reaction, 2.5g of calcium carbonate react completely with excess hydrochloric acid to produce 600cm³ of carbon dioxide gas in 1.5 minutes. Find the rate of reaction in term of
a. decreasing mass of calcium carbonate
b. increasing volume of carbon dioxide gas produced
Answer:
a.
Change of the amount of reactant=-2.5g Tima taken for the change=1.5minute=90s Rate of Reaction = -2.5g / 90s = 0.027gs-1
b.
Change of the amount of product
=600cm3 Tima taken for the change
= 1.5minute = 90s
Rate of Reaction
= 600cm3/90s
=6.7cm3s-1