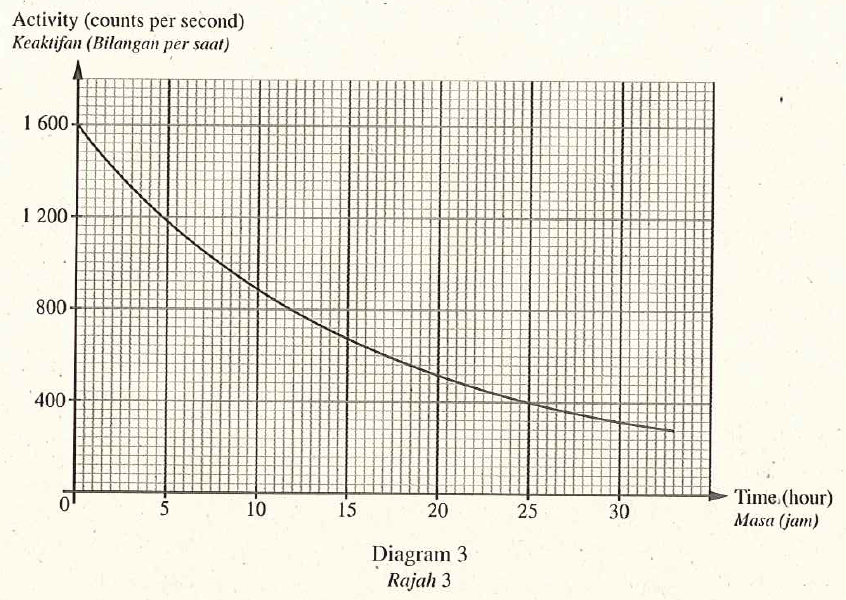
Diagram 3 shows the activity-time graph of radioisotope Potassium-42. This radioisotope emits beta particles and is used as a tracer to measure the quantity of salt in human body.
- State one characteristic of beta particles. [1 mark]Negatively charged
- Based on Diagram 3 , determine the half-life of Potassium-42. Show on the graph how you determine the half-life of Potassium. [2 mark]12 hours
- Based on the half life, state why Potassium-42 is suitable to be used as the tracer. [1 mark]Potassium-42 has short half-life, hence overexposure does not occur.
- Calculate the time taken for radioistope Potassium-42 to reduce \(\frac{1}{8}\) of its initial activity. [2 marks]\(1 \underset{ T _{\frac{1}{2}}}{\longrightarrow} \frac{1}{2} \underset{ T _{\frac{1}{2}}}{\longrightarrow} \frac{1}{4} \underset{ T _{\frac{1}{2}}}{\longrightarrow} \frac{1}{8}\)
The time taken for the activity of the potassium- 42 becomes \(\frac{1}{8}\) of its initial activity.
\[
\begin{aligned}
&=3 T _{\frac{1}{2}} \\
&=3 \times 12 \text { hours } \\
&=36 \text { hours }
\end{aligned}
\]