Structure Question 2:
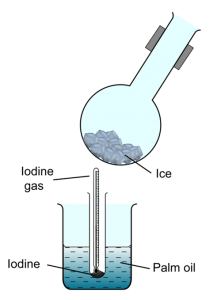
An experiment is conducted to study the change of state of iodine. Some powder of solid iodine is heated as shown in the Figure above, the black solid iodine changes into a purple gas at 125°C. The purple gas is then cooled by a round bottom flask that fill with ice.
Answer:
- State the process of change of state demonstrated by iodine at 125°C.Sublimation
- What can be observed at part R?
Some black powder form at the bottom of the flask.
- What is the name of the process when iodine gas turns into iodine solid again?
Reverse/inverse sublimation
- Explain why palm oil is used in the experiment instead of water.
Because iodine sublime at 125°C, the temperature which is higher than the boiling point of water.
- Name two other substances which also sublime at atmospheric pressure (1atm).
Ammonium chloride, carbon dioxide, naphthalene.