Contact Process
Sulphuric Acid is Manufactured in Industry
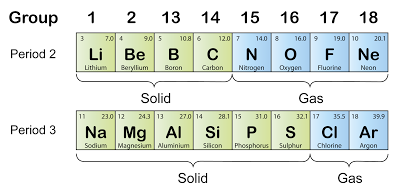
- Sulphuric acid, H2SO4 is manufactured in the industry through Contact Process.
- The raw materials used are sulphur, air and water
- The Contact process consists of four stages.
Stage 1
- Molten sulphur is burnt in dry air to produce sulphur dioxide
- The gas produced is then purified and cooled.
S + O2 → SO2 - Sulphur dioxide can also be produced by burning metal sulphides such as lead(II) sulphide or zinc sulphide in dry air.
2PbS + 3O2 → 2PbO + 2SO2
Stage 2
- In a converter, sulphur dioxide and excess oxygen are passed through vanadium(V) oxide.
- vanadium(V) oxide act as a catalyst to expedite the process.
- The optimum condition for the maximum amount of product are as follow:
- Temperature: 450 – 500 °C
- Pressure: 2 – 3 atm
- About 99.5% of the sulphur dioxide, SO2 is converted into sulphur trioxide, SO3 through this reversible reaction.
Stage 3
Sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum (H2S2O7).
SO3 + H2SO4 → H2S2O7
Stage 4
The oleum, H2S2O7 is then diluted with water to produce concentrated sulphuric acid, H2SO4 in large quantities.
H2S2O7 + H2O → 2H2SO4
Note:
- The two reactions in the third and fourth stages are equivalent to adding sulphur trioxide, SO3 directly to water.
SO3 + H2O→ H2SO4 - However, this is not done in the industry because sulphur trioxide, SO3 reacts too violently with water.
- This produces a lot of heat and a large cloud of sulphuric acid, H2SO4 mist.
- The mist is corrosive, pollutes the air and is difficult to condense.