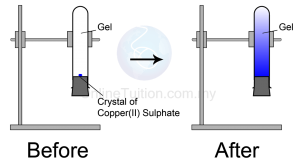
Structure Question 1:
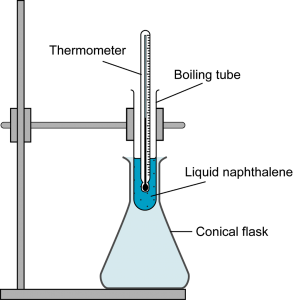
The above diagram shows the apparatus used in an experiment to determine the freezing point of liquid naphthalene. The liquid naphthalene is cooled from 100°C in a conical flask and it is stirred continuously with a thermometer. The temperature of liquid naphthalene is recorded in every 30 seconds. The results obtained are tabulated below:
| Time (s) | 0 | 30 | 60 | 90 | 120 | 150 |
| Temperature (oC) | 100 | 93 | 85 | 78 | 78 | 78 |
| Time (s) | 180 | 210 | 240 | 270 | 300 |
| Temperature (oC) | 78 | 60 | 43 | 25 | 25 |
Answer:
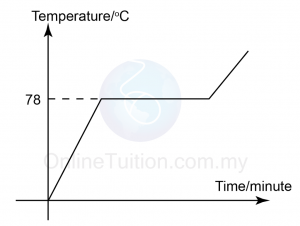
- Draw a graph of temperature against time for the cooling of liquid naphthalene. [2 marks]
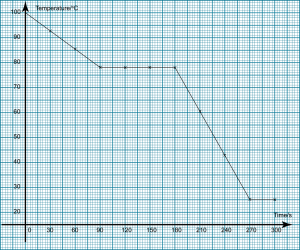
(Click on the image to enlarge) - Determine the freezing point of liquid naphthalene from the graph.78°C
- c.What is the physical state of naphthalene at
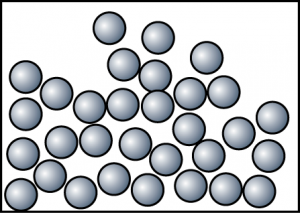
- 60sliquid
- 120sliquid and solid
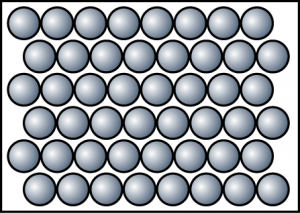
- 180ssolid
- 60s
- Draw the particles arrangement of naphthalene at c.i. and c.iiic. i.
c. ii.
- Explain why the temperature remains constant between 3 and 6 minutes?
From the time 90 second to 180 seconds, naphthalene is freezing. During freezing, bonds are formed in between the molecules and energy is released. The energy lost to the surrounding is compensated by the energy released from the formation of the bonds. - Explain why the boiling tube is placed inside a conical flask during the cooling process.
To ensure constant cooling at a slow rate for naphthalene. This can avoid supercooling. - Give a reason why naphthalene needs to be stirred continuously during the process?
To avoid supercooling. - Will the melting point of naphthalene differ if it is contaminated by other substance?
Yes - Sketch a graph obtained when solid naphthalene is heated from room temperature (25°C) to 100°C.